About ZULRESSO
ZULRESSO may offer rapid results
in 60 hours (2.5 days)
ZULRESSO® (brexanolone) was proven effective in the treatment of postpartum depression (PPD) in 2 Phase III clinical trials.
Phase III clinical studies of ZULRESSO showed a statistically significant reduction in PPD severity in 60 hours (2.5 days)
ZULRESSO was studied in 2 multicenter, randomized, double-blind, placebo-controlled studies in women aged 18 to 45 years with PPD who were <6 months postpartum at screening and met the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria for a major depressive episode with onset of symptoms in the third trimester or within 4 weeks of delivery.
Individual results may vary.
HAM-D=Hamilton Depression Rating Scale;
LS=least squares.
*2.5 days=Hour 60.
Patients received a 60-hour continuous intravenous (IV) infusion of ZULRESSO or placebo and were then followed for 4 weeks. Baseline oral antidepressant use was reported for 23% of patients.
- In Study 1 and Study 2, the primary endpoint was the mean change from baseline in depressive symptoms as measured by the HAM-D total score at the end of the infusion (Hour 60)
- In both placebo-controlled studies, titration to the recommended target dose of ZULRESSO 90 mcg/kg/hour was superior to placebo in improvement of depressive symptoms
- In a group of 38 patients in Study 1, a ZULRESSO titration to a target dose of 60 mcg/kg/hour was superior to placebo in improvement of depressive symptoms
STUDY 11
SEVERE
Recommended target dosage:
90 mcg/kg/hour arm
Baseline: mean HAM-D score of 28.4
- LS mean change from baseline: -17.7
- Difference from placebo arm: -3.7 (P=0.0252)‡
- 62.3% reduction in mean HAM-D score from baseline at Hour 60 (n=41) vs 49% with placebo (n=43, P=0.0252)
Target dosage:
60 mcg/kg/hour arm
Baseline: mean HAM-D score of 29.0
- LS mean change from baseline: -19.5
- Difference from placebo arm: -5.5 (P=0.0013)‡
Placebo arm
Baseline: mean HAM-D score of 28.6
- LS mean change from baseline: -14.0
‡Intention-to-treat population.
STUDY 21
MODERATE
Recommended target dosage:
90 mcg/kg/hour arm
Baseline: mean HAM-D score of 22.6
- LS mean change from baseline: -14.6
- Difference from placebo arm: -2.5 (P=0.0160)‡
- 64.6% reduction in mean HAM-D score from baseline at Hour 60 (n=51) vs 53.3% with placebo (n=53, P=0.0160)
Placebo arm
Baseline: mean HAM-D score of 22.7
- LS mean change from baseline: -12.1
Select Important Safety Information
WARNING: EXCESSIVE SEDATION
AND SUDDEN LOSS OF CONSCIOUSNESS
Patients treated with ZULRESSO are at risk of excessive sedation or sudden loss of consciousness during administration. Because of the risk of serious harm, patients must be monitored for excessive sedation and sudden loss of consciousness and have continuous pulse oximetry monitoring. Patients must be accompanied during interactions with their child(ren).
Because of these risks, ZULRESSO is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the ZULRESSO REMS.
ZULRESSO-treated patients experienced rapid improvement of depressive symptoms vs placebo1
Change from baseline in HAM-D total score over time in Study 1 with the recommended target dosage of ZULRESSO (90 mcg/kg/hour)1,3
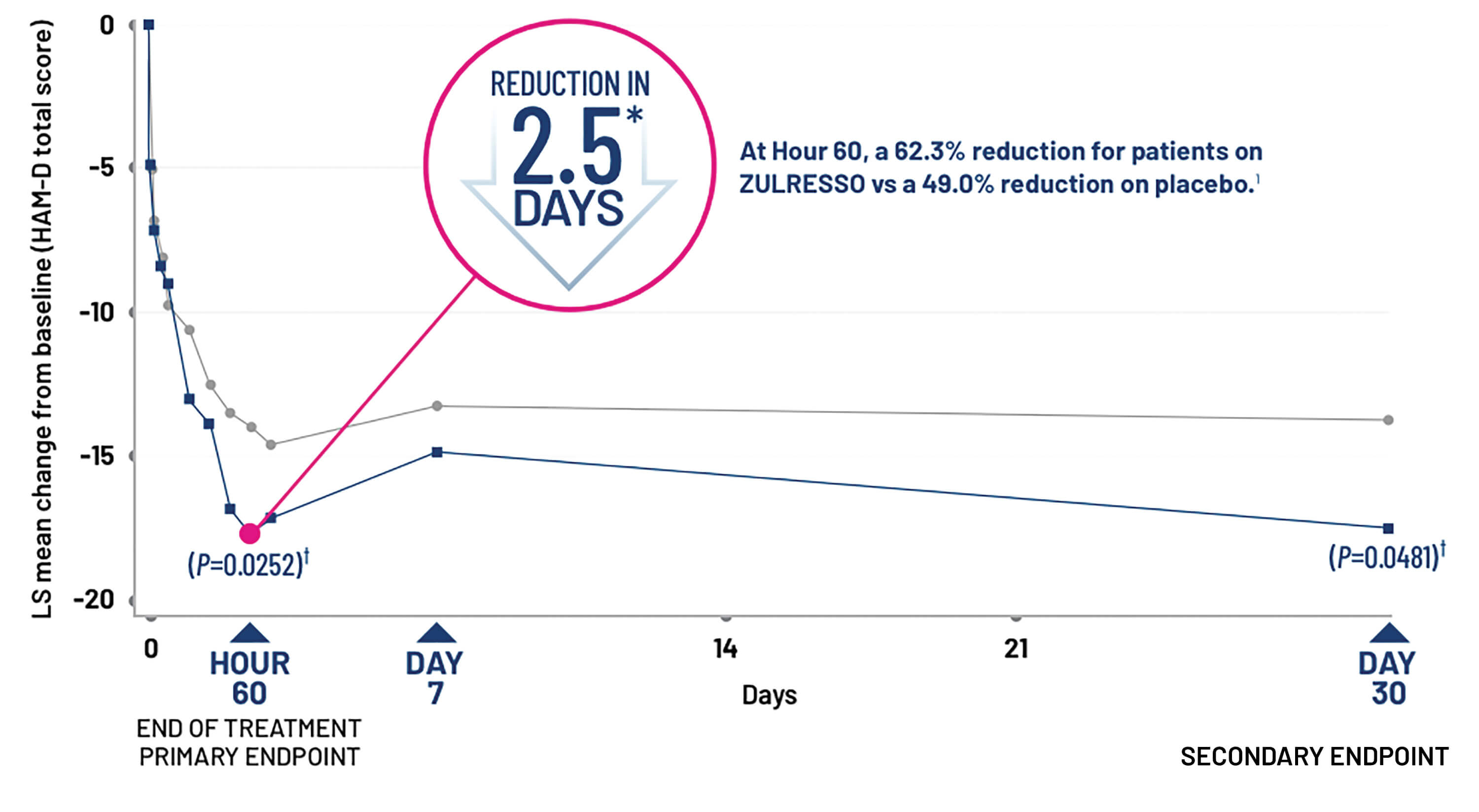
A similar therapeutic effect was also observed in the 60 mcg/kg/hour arm.
*2.5 days=Hour 60.
†Statistically significant after multiplicity adjustments.
‡Intention-to-treat population.
Individual results may vary.
HAM-D=Hamilton Depression Rating Scale; LS=least squares.
Durable therapeutic effect1,3
HAM-D total score did not return to baseline by Day 30 in both Study 1 and Study 2. A prespecified secondary efficacy endpoint was the mean change from baseline in HAM-D total score at Day 30.
- In the overall Study 1 population, significantly greater reduction in HAM-D total score with ZULRESSO vs placebo was observed at Day 30 (P≤0.05)
- In Study 1, the 60-mcg/kg/hour arm was significant at Hour-60 and maintained significance vs placebo at Day 30
- In Study 2, the 90 mcg/kg/hour arm maintained therapeutic effect to a similar magnitude at Day 30, but did not show a greater difference vs placebo
Risk of suicidal thoughts and behaviors: ZULRESSO does not directly affect monoaminergic systems. Because of this and the comparatively low number of exposures to ZULRESSO, the risk of developing suicidal thoughts and behaviors with ZULRESSO is unknown.1
Consider changing the therapeutic regimen, including discontinuing ZULRESSO, in patients whose depression becomes worse or who experience emergent suicidal thoughts and behaviors.1
