About ZULRESSO
ZULRESSO safety profile
Learn about the safety profile of ZULRESSO® (brexanolone) and consider if it may be right for your patients.
Excessive sedation and sudden loss of consciousness1
In clinical studies in adults, ZULRESSO caused sedation and somnolence that required dose interruption or reduction in some patients during the infusion (5% of ZULRESSO-treated patients compared to 0% of placebo-treated patients). Some patients were also reported to have loss of consciousness or altered state of consciousness during the ZULRESSO infusion (4% of the ZULRESSO-treated patients compared with 0% of the placebo-treated patients). Time to full recovery from loss or altered state of consciousness, after dose interruption, ranged from 15-60 minutes in clinical studies in adults. A healthy 55-year-old man participating in a cardiac repolarization study experienced severe somnolence and <1 minute of apnea while receiving two times the maximum recommended dosage of ZULRESSO (180 mcg/kg/hour).
In an open-label clinical study in 20 patients ages 15 to 17 years, one patient experienced dizziness and loss of consciousness. All patients with loss of or altered state of consciousness recovered with dose interruption.
There was no clear association between loss or alteration of consciousness and pattern or timing of dose. Not all patients who experienced a loss or alteration of consciousness reported sedation or somnolence before the episode.
During the infusion
During the infusion, monitor patients for sedative effects every 2 hours during planned, non-sleep periods. Immediately stop the infusion if there are signs or symptoms of excessive sedation.
After symptoms resolve, the infusion may be resumed at the same or lower dose as clinically appropriate.
Immediately stop the infusion if pulse oximetry reveals hypoxia. After hypoxia, the infusion should not be resumed.
Caution patients
Patients should be cautioned against engaging in potentially hazardous activities requiring mental alertness, such as driving, after infusion until any sedative effects of ZULRESSO have dissipated. Patients must be accompanied during interactions with their child(ren) while receiving the infusion because of the potential for excessive sedation and sudden loss of consciousness.
Concomitant use
Concomitant use of opioids, antidepressants, or other CNS depressants such as benzodiazepines or alcohol may increase the likelihood or severity of adverse reactions related to sedation.
ZULRESSO REMS1
ZULRESSO is available only through a restricted program under a Risk Evaluation and Mitigation Strategy called the ZULRESSO REMS because excessive sedation or sudden loss of consciousness can result in serious harm.
Suicidal thoughts and behaviors1
Risk with antidepressants
In pooled analyses of placebo-controlled trials of chronically administered antidepressant drugs (selective serotonin reuptake inhibitors [SSRIs] and other antidepressant classes) that included approximately 77,000 adult patients and 4,500 pediatric patients, the incidence of suicidal thoughts and behaviors in antidepressant-treated patients age 24 years and younger was greater than in placebo-treated patients.
Variation in risk
There was considerable variation in risk of suicidal thoughts and behaviors among drugs, but there was an increased risk identified in young patients for most drugs studied. There were differences in absolute risk of suicidal thoughts and behaviors across the different indications, with the highest incidence in patients with major depressive disorder (MDD).
Considerations for ZULRESSO
ZULRESSO does not directly affect monoaminergic systems. Because of this and the comparatively low number of exposures to ZULRESSO, the risk of developing suicidal thoughts and behaviors with ZULRESSO is unknown. Consider changing the therapeutic regimen, including discontinuing ZULRESSO, in patients whose depression becomes worse or who experience emergent suicidal thoughts and behaviors.
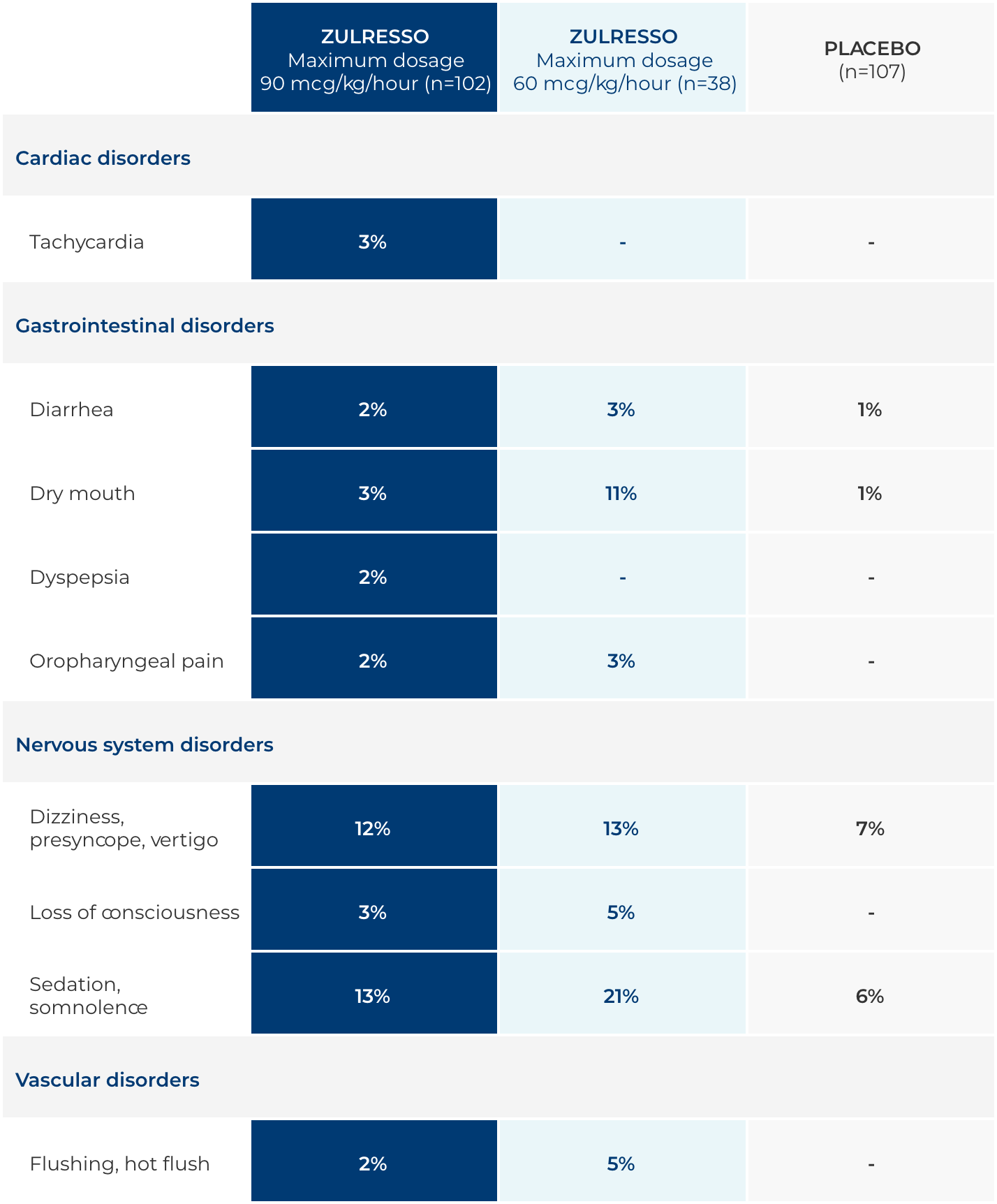
ZULRESSO adverse reactions1
Adverse reactions reported in ≥2% of ZULRESSO-treated adult patients and greater than in placebo-treated patients during the 60-hour treatment period
Patients 15 to 17 Years
The safety of ZULRESSO was evaluated in an open-label study in patients 15 to 17 years. A titration to a target dosage of 90 mcg/kg/hour was evaluated in 20 patients with postpartum depression. Patients were then followed for 4 weeks. Adverse reactions reported in the clinical study were generally similar to those observed in clinical studies of ZULRESSO in adults with PPD.
The data described in the table reflect exposure to ZULRESSO in 140 adult patients across 3 studies.1-3
Most common adverse reactions
The most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were sedation/somnolence, dry mouth, loss of consciousness, and flushing/hot flush.
Discontinuation
In the pooled placebo-controlled studies, the incidence of patients who discontinued due to any adverse reaction was 2% of ZULRESSO-treated patients compared to 1% of placebo-treated patients. The adverse reactions leading to treatment discontinuation in ZULRESSO-treated patients were sedation related (loss of consciousness, vertigo, syncope, and presyncope) or infusion-site pain.
Interruption or reduction
In the pooled placebo-controlled studies, the incidence of patients who had an interruption or reduction of the dosage due to any adverse reaction was 7% of ZULRESSO-treated patients compared to 3% of placebo-treated patients. The adverse reactions leading to dose reduction or interruption in ZULRESSO-treated patients were sedation related (loss of consciousness, syncope, somnolence, dizziness, fatigue), infusion-site events, changes in blood pressure, or medication error due to infusion pump malfunction. Three ZULRESSO-treated patients who had a dosage interruption because of loss of consciousness subsequently resumed and completed treatment after resolution of symptoms; two patients who had dosage interruption because of loss of consciousness did not resume the infusion.
Use in specific populations
Pregnancy: Based on findings from animal studies of other drugs that enhance GABAergic inhibition, ZULRESSO may cause fetal harm.
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antidepressants, including ZULRESSO, during pregnancy. Healthcare providers are encouraged to register patients by calling the National Pregnancy Registry for Antidepressants at 1-844-405-6185 or visiting online at https://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/antidepressants/
Pediatric Use: Safety and effectiveness of ZULRESSO for the treatment of PPD have been established in patients 15 to 17 years. Use of ZULRESSO in this population is supported by evidence from adequate and well-controlled studies in adults with PPD, pharmacokinetic data in adults and patients 15 to 17 years, and safety data in patients 15 to 17 years.
The safety and effectiveness of ZULRESSO in patients less than 15 years of age have not been established.
Renal Impairment: Avoid use of ZULRESSO in patients with end-stage renal disease (ESRD) with eGFR of <15 mL/minute/1.73 m2 because of the potential accumulation of the solubilizing agent, betadex sulfobutyl ether sodium.
Breastfeeding considerations1
There are no data on the effects of ZULRESSO on a breastfed infant.
Lactation study information
- A study was conducted in 12 healthy adult lactating women treated with intravenous ZULRESSO according to the recommended 60-hour dosing regimen (maximum dosage was 90 mcg/kg/hour)
- The study indicated that brexanolone is transferred to breastmilk in nursing mothers
- Concentrations of ZULRESSO in breast milk were at low levels (<10 ng/mL) in >95% of women by 36 hours after the end of the infusion of ZULRESSO
- The calculated maximum relative infant dose for ZULRESSO during the infusion is 1% to 2% of the maternal weight-adjusted dosage
Risk considerations
- ZULRESSO has low oral bioavailability (<5%) in adults; therefore, infant exposure is expected to be low
- Available data on the use of ZULRESSO during lactation do not suggest a significant risk of adverse events to breastfed infants from exposure to ZULRESSO
- The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ZULRESSO and any potential adverse effects on the breastfed child from ZULRESSO or from the underlying maternal condition
Discuss the potential benefits and risks of breastfeeding during the infusion with your patients.
